This past week Michael started his fourth year teaching! Super exciting! The two days before the kids arrived, he and his co-teacher had a conversation about smells and scented gel beads! You know, those types of things that are considered odor eliminators. He came home asking how they work and I could only explain diffusion to a certain extent regarding them! Boom! The perfect topic for #ScienceSunday!
The easiest way to look at smells is obviously to think of the stinky ones LOL. They are all made of molecules. A molecule is the smallest fundamental unit that can be involved in a chemical reaction. They are made up of a group of atoms. When we smell something, good or bad, we inhale through our nose where our olfactory receptor neurons detect the smell. The neuron receptors transmit the information to the olfactory bulb at the back of the nose. The bulbs have sensory receptors that are part of the brain that can send signals to the limbic system (where our memories and emotions are) and the neocortex (controls conscious thoughts). This is why when we smell something we recognize, we automatically think of exactly where we last smelled it! Pretty awesome!!
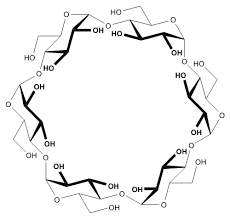
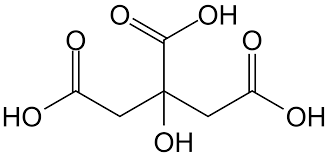
Most of the odor eliminators are made up of two important compounds; cyclodextrin and citric acid. Both of these can be naturally derived. Cyclodextrin is a starch molecule made from corn while citric acid can be found in citrus fruits.
Cyclodextrin, one of the odor eliminators, is a donut shaped molecule, so when a “stinky” molecule (like the ones from rotten eggs for example) which is usually spherical, runs into the donut shaped molecule, it gets trapped in the center. So at this point, you are only be able to smell the odor eliminator molecule if it has a fragrance or you will not smell the “stinky” molecule at all. These are known as odor trappers; very fitting LOL!
Citric acid, another odor eliminator, is known as an odor neutralizer which means it eliminates smell by balancing the pH of the “stinky” molecule to that of the pH of water which is 7. This makes the “stinky” molecule odorless. Since it is more acidic it is able to balance out a usually basic “stinky” molecule.
Then there are odor converters that are reactive perfumatic ingredients capable of changing all types of odors. They are pliable molecules that hold onto the “stinky” molecule and will not let it go until it converts itself into something that smells less pungent.
Lastly are odor magnets which are molecules that have perfume release factors. These factors will only let go if their sensors attract and grab onto an odor molecule. When an odor molecule is picked up, the perfume factors are released.
These various molecules can be found in all types of odor sprays like Febreeze, Glade, Renuzit, and others you may know. And it happens at a microscopic level which is insane!
So the next time you go #2 and you spray that odor spray, you’ll know exactly how you are masking that smell!!
Xox, Z