With Easter around the corner, I figured we should take a deeper look at a tradition we have all participated in: Easter egg dyeing! I talked to some coworkers about their experiences with Easter egg dyeing and they all mentioned adding vinegar to the color mixtures to make a more vibrant color. Okay sure that works but how does it work?? Also what are the main differences between using synthetic dyes and natural dyes? Let’s take a look! 🙂
So before we get into the chemistry topics, please make sure if you want to make colorful Easter eggs boil your eggs first so you don’t make a mess. LOL, no one wants to clean that up.
Basic egg dyeing consists of combining boiling water with vinegar and food coloring. The food coloring we use (usually McCormick brand) is made of water, propylene glycol, FD&C yellow 5, FD&C red 40, FD&C blue 1, FD&C red 3, and 0.1% propylparaben as a preservative. FD&C is the acronym for Food, Drug, and Cosmetic Act which means they are regulated food dyes. These are synthetic dyes made of organic molecules. You dunk the hard boiled egg into the dye and it absorbs into the shell.
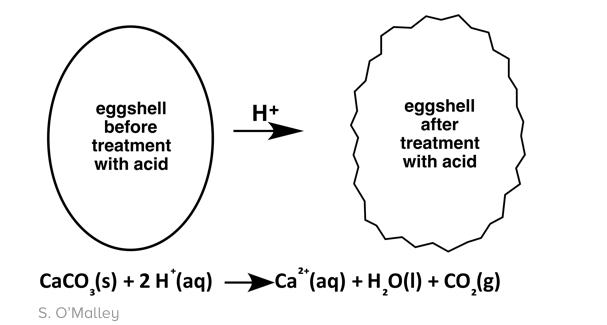
We add vinegar to the solution for a few reasons. The most important is the fact that when vinegar is added to the dye, the color becomes way more vibrant. When you first drop the egg into the solution, the shell reacts with the acetic acid (vinegar) forming bubbles of carbon dioxide gas. This causes the shell to start to break down allowing a larger surface area to form which means more of the egg gets exposed to the dye. The proteins in the eggshell underlayer become positively charged (protonated) by the acetic acid (vinegar). When that happens, the surface of the egg has positive charge which attracts the negatively charged dye making it stick more. The same process can be used with other acids like orange juice or soda but using vinegar is the best because of its potency and strength as an acid. Plus it has no color!
When we dye the eggs what we see is the light that is reflected from the surface of the egg. This happens when light hits the surface. A certain wavelength of colors get absorbed while others are reflected. Dyeing eggs changes the molecular composition of the surface therefore changing the way light gets absorbed or reflected.
Now onto the dyes themselves! McCormick food dyes are the most common used but you can make natural dyes using fruits, vegetables, and spices you can find around the house. If you want to try out a blue/purple color boil some red cabbage, red/orange colors can come from beets, and yellows/orange colors can come from turmeric plants.
Once Easter comes around, I will share with you my family’s traditions and how they’ve changed over the years!
Xoxo,
Z