Almost every piece of clothing that we pick up at a store is made with some percentage of cotton. But if everything is made of cotton, how does the world keep up with production?
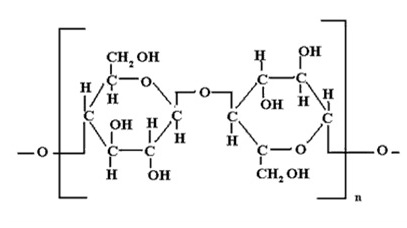
Cotton is a natural cellulose fiber used in clothing, coffee filters, and other products. It grows in a protective capsule into a soft and fluffy fiber. It is about 88 to 96% pure cellulose with trace amounts of proteins, fats, ash, and sometimes pigment. Cellulose is a polymer of beta glucose with cotton configured as two repeating units of cellulose connected at carbon 1 and carbon 4. As the polymer repeats, it forms a flat chain that is perfect for a durable fiber. Within the molecule, there are reactive hydroxyl groups that allow for modification during any type of customization.
Cotton is described by three physical properties that determine its quality. Before modern technology these properties were looked at by hand. Now, they are determined by instruments calibrated to the properties.The three properties are: micronaire, length, and strength. Micronaire is the measure of air flow to determine fineness of the fiber measured by forcing air on the fiber and measuring the resistance. Length is determined by the various types of cotton that is grown. Strength of the type of cotton is measured by the force required to break a bundle of fibers.
When the plant is plucked, there is a coating of wax on the outside which can be used for unaesthetic products. For the aesthetic cotton, it must be scoured and bleached. In a normal scouring process, the fiber is opened and cleaned followed by an alkali (basic) solution (most likely sodium hydroxide) application and reaction, rinsing, bleach (hydrogen peroxide solution) application and reaction, rinsing, finishing application, drying, and bale formation. There is another method that is more environmentally friendly and saves water by combining the scouring and bleaching process in one step which reduces the rinsing to one step. This is known as continuous scouring and bleaching. After this process, the cotton is considered acceptable the US Pharmacopeia standards. I had no idea that USP was involved in this process. That’s freaking cool! When the cotton has been bleached, a finishing agent such as butoxyethyl stearate will be applied to give the fiber lubrication without sacrificing absorbency.
At this point, the cotton can be used to manufacture all types of goods and products. It’s so interesting to see how much goes into taking a protected capsule and turning into a byproduct to be used for an end product that we wear or use in our homes.
Thanks for reading!!
Z